What is determinism?
Determinism is the concept that the physical world that we live in is wound like a clock. The concept says that if we would know every law that governs the universe, and we have the computational power to compute those laws; then, we may know the future with 100% certainty.
Bad news is…
This universe is non-deterministic. We know this, now, with great certainty, and with many experiments and many successful models that have shown, so far, that this is true. In fact, Einstein had fought his last 20 years, before his death, to disprove this fact, and he failed.
Will someone else disprove it? A Nobel prize awaits this Genius, if he could do it!
Main question of the article
What if an observer lives outside our universe and monitors the universe from the outside, and knows the internal parameters that control our universe. Will he be able to determine the future infinitely precisely?
It’s a very complicated question in fact to think about. However, in order to answer the question, we have to understand what is non-deterministic in our universe.
How can we tackle this question?
In order to understand the answer to this question, we have to understand what it is that we have to predict. And understand why uncertainty shows up in the first place.
Where does uncertainty come from?
Uncertainty comes mainly from the fact that we deal with a world governed by some classical parameters. For example, we deal with energy and position. Those parameters, if known very well, describe our systems very accurately.
In other words, in the simplest form, if we know the positions and energies of a set of particles; we may, as well, predict the future and dynamics of the system very accurately.
What is it that we’re uncertain about in our universe?
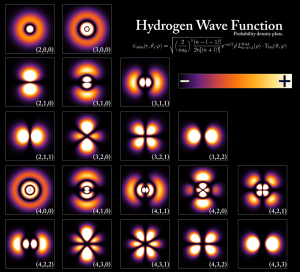
Here comes the problem. When we go to the microscopic level of particles, we find that the macroscopic (large scale) description of systems is very different. In quantum mechanics (QM), systems are defined by the so called “wave packets”. They’re not anymore “objects” like we thought of them before.
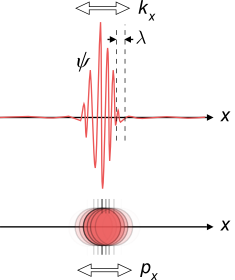
The problem is the transition
Our particles are described by wave packets in the microscopic level. But in our world, we don’t deal with wave packets. We deal with particles. We deal with well defined energies and well defined positions. Therefore, we need a transformation that will take our wave packet from its wavy picture, to a picture that is compatible with our classical observations. This transformation is called in QM the expectation value. When performing this transition, there is no way we can do it without uncertainty. For example, in the particle seen in the picture, we can never, ever, define a single point that characterizes the position of the particle. There’s no position! The particle is smeared over a volume of space. Therefore, the transformation from the QM picture, with wavy properties, to the classical picture, is the cause of uncertainties.
Conclusion
The reason of non-determinism in this world is not that we don’t know the characteristics of a particle in its wave nature. The main problem causing uncertainty to appear is that the description of particles in their microscopic form is always coherent with uncertainties, due to the incompatibility of the world views when taking the step from the microscopic world to the classical world.
Answering the main question: Will someone outside the universe, who knows the parameters of those wave packets, be able to predict the future with 100% certainty? The answer is NO. Because the fact that a transformation from the wavy form to the classical form contains uncertainties is not related to our physical world. It’s rather related to the mathematical nature of this transformation, which inherently will cause uncertainties to appear, independent of the knowledge of the entity performing this transformation.